An electrical conductor is a substance that has a low resistivity and easily conducts electric current.. The presence of a large number of freely moving charged particles in a conductor is called a carrier.. Under the action of an external electric field, carriers move directionally, creating an apparent current. So, what is the best conductor of electricity?

Table of Contents
- Types of Electrical Conductor
- What Metal Is the Best Conductor of Electricity?
- Factors Affecting Electrical Conductivity
- Electrically Conductive Materials
Types of Electrical Conductor
Solid conductors
Metals and graphite are the most common solid conductors. Los valence electrons of the outer layers of metals and graphite are easily detached from the core. and become free electrons, that form the carriers of electrical conductivity.
The concentration of free electrons in metals and graphite is very large., around 10^22 per cubic centimeter. The resistivity of metals and graphite is, so, very small and the electrical conductivity very large.
Liquid conductors
Electrolyte solutions are also conductors, whose carriers are positive and negative ions. It has been shown that most pure liquids, although they can dissociate, they do it to a small degree and, so, they are not drivers. Nevertheless, if a little electrolyte is added, the ion concentration is greatly increased, which causes the resistivity to drop considerably.
This is because the concentration of carriers in the electrolyte is much lower than that of metals.. Besides, ions have a great interaction with the surrounding medium, which makes its mobility in the external electric field much less.
Conductive Gases
Ionized gases can also conduct electricity and their carriers are positive and negative electrons and ions.. As a rule, gases are good insulators. Gas molecules can be dissociated with the help of external manipulations such as heating or X-ray irradiation, gamma rays or ultraviolet light. The ionized gas can then become a conductor..
The conductivity of ionized gases is highly dependent on the applied voltage and is often accompanied by physical processes such as sound generation and luminescence.. Ionized gases are often used in the manufacture of electric light sources..
What Metal Is the Best Conductor of Electricity?
The concentration of free electrons in metals is large.. So, the conductivity of the metallic conductors is usually greater than that of other conductive materials. The resistivity of metallic conductors generally decreases as the temperature drops.. At very low temperatures, the resistivity of some metals and alloys almost disappears and becomes superconducting.
Regarding electrical conductivity, the highest is that of silver, followed by copper and gold. Silver also has the highest thermal conductivity and light reflectivity of all metals..
Why is silver the best conductor of electricity?? It has to do with its chemical valency and its crystalline structure.. Compared to other metals, silver's electrons move more freely.
Nevertheless, although silver is the best material conductor, copper and aluminum are used more in electrical applications. This is because copper is cheaper and has a higher resistance to corrosion.. At payment, instead, has reduced conductivity on its outer surface when it loses its luster.
Table of the Order of Conductivity of Metals
How the size and shape of a substance affect its electrical conductivity, All samples are assumed to be the same size.. The order of common live metals from most conductive to least conductive is as follows.
Plata > Copper > Oro > Aluminum > Zinc > Nickel > Brass > Bronze > Hierro > Platinum > Carbon Steel > Lead > Stainless steel
Factors Affecting Electrical Conductivity
The electrical conductivity of a material can be influenced by external conditions and, usually, there are five main factors.
- Temperature: The change in temperature of any conductor will modify its electrical conductivity. In general, the increase in temperature causes thermal excitation of the atoms and decreases the conductivity, while resistivity increases. This relationship is usually linear, except at low temperatures.
- impurities: Adding impurities to a conductor reduces its conductivity.. For example, unrefined silver is not as good a conductor as pure silver. Rusty silver is not as good as shiny silver. Impurities affect conductivity because they prevent the free movement of electrons..
- Crystal structure and phases: If there are different phases in the material, electrical conductivity is slightly reduced at the interface. The way the material is handled also affects its electrical conductivity..
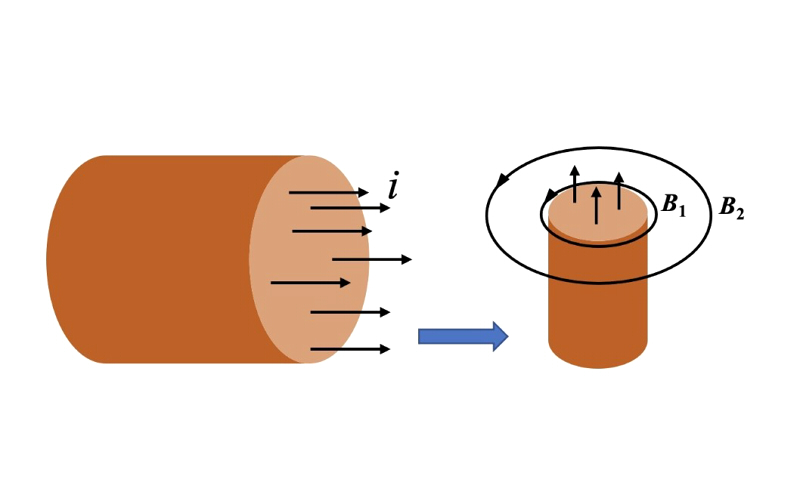
- Electromagnetic field: When an electric current flows through a conductor, generates its own electromagnetic field. The magnetic field is perpendicular to the electric field.. The external electromagnetic field creates a magnetic resistance that slows down the flow of current.
- Frequency: The number of oscillation cycles per second that the alternating current completes is its frequency.. From a certain level, high frequencies cause current to flow around a conductor without going through it (film effect). As there are no oscillations, the skin effect does not occur with direct current.
Electrically Conductive Materials
Los electrically conductive materials are materials used to transmit electrical current with only a small loss of energy. They are mainly represented by electrical wires and cables.
With the development of the electricity industry, conductive coatings are also widely used, transparent conductive materials and adhesives. The basic properties of conductive materials are characterized by their resistivity.

The metallic conductors used in electrical wires and cables are mainly copper., aluminum and its alloys. Copper as a conductive material is mainly electrolytic copper, with a copper content of 99,97% al 99,98%. The presence of metallic impurities and oxygen can reduce the electrical conductivity of copper conductors..
Aluminum wires have low electrical conductivity compared to copper wires.. Nevertheless, its weight is light and its relative density is only 1/3 of the copper. This is one of the main advantages of aluminum conductors. Mainly used as power and distribution line. For the high voltage cables of more than 160 kV, aluminum conductors reinforced with steel or aluminum alloy wires are often used.

